

Semi-dry electroblotting (Semi-dry transfer) With increasing time, however, there is a risk of over-transfer (stripping, blow through) of the proteins through the membrane, especially for lower molecular weight (<30 kDa) proteins when using membranes with a larger pore size (0.45 µm). The transfer efficiency improves with increased transfer time and is better, in general, for lower molecular weight proteins than higher molecular weight proteins. Transfer efficiencies of 80–100% are achievable for proteins between 14–116 kDa. A high field option exists for transferring a single gel, which may bring transfer time down to as little as 30 minutes, but it requires the use of high voltage (up to 200 V) or high current (up to 1.6 A) and a cooling system to dissipate the tremendous heat produced. Multiple gels may be electrotransferred in the standard field option, which is performed either at constant current (0.1 to 1 A) or voltage (5 to 30 V) from as little as 1 hour to overnight. The supported gel sandwich is placed vertically in a tank between stainless steel/platinum wire electrodes and the tank is filled with transfer buffer. The gel is then placed in the “transfer sandwich” (filter paper-gel-membrane-filter paper), cushioned by pads and pressed together by a support grid.
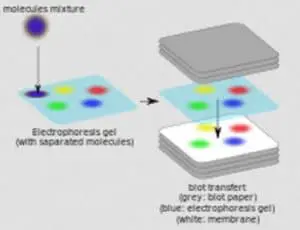
When performing a wet transfer, the gel is first equilibrated in transfer buffer. The efficiency of protein transfer can be affected by the chemistry, thickness of the gel, the molecular weight of the proteins being transferred, the type of membrane and transfer buffers used, and the transfer method. In addition to the challenges of immunodetection in the protein blotting workflow, the transfer of proteins from a gel matrix to a membrane is a potential hurdle. An appropriate method is then used to detect the localized probe to document the location and relative abundance of the target protein. The transferred protein is then probed sequentially with antibodies and detection probe (e.g., enzyme, fluorophore, isotope).
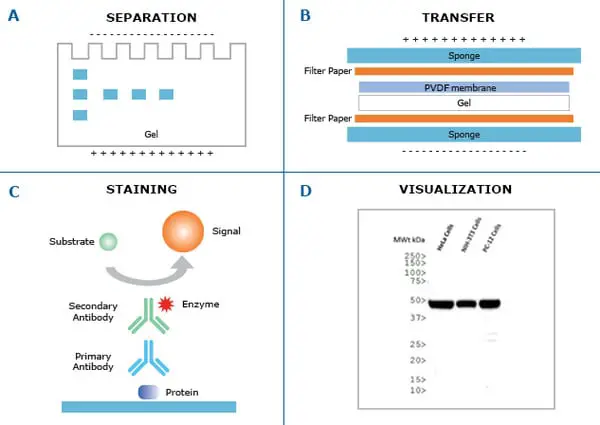
After electrophoresis, the separated proteins are transferred, or "blotted", onto a solid support matrix, usually a nitrocellulose or polyvinylidene difluoride (PVDF) membrane. The first step in a western blotting procedure is to separate the proteins in a sample by size using denaturing gel electrophoresis (i.e., sodium dodecyl sulfate polyacrylamide gel electrophoresis or SDS-PAGE) or native PAGE.


 0 kommentar(er)
0 kommentar(er)
